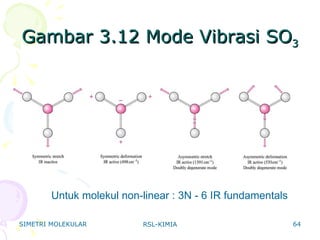
38 label all bonds in so2
Label All Bonds In So2 - How Many Covalent Bonds Are Present In An So2 ... Label All Bonds In So2 - How Many Covalent Bonds Are Present In An So2 Molecule Quora. 18.05.2020 · white crystals or crystalline powder. Label each variable in the ideal gas law with the property it represents. This function sets all amide dihedral angles to 180 degrees and provides the option for energy minimization … Label all bonds in CH2Br2. Label all bonds in SO2. Label ... - Transtutors Label all bonds in CH2Br2 Label the diagram by dragging the labels to the appropriate targets. Note that not all labels will be used. o C (sp) H (s) o C (sp') Br (s) C (p) H (p) C (p) Br (p) C (sp) H (p) o C (sps) Br (p) C (sps) Br (p) reset help Expert's Answer Solution.pdf Next Previous Q: View Answer Q: Q: Posted 2 months ago Q:
What orbitals are used to form the 10 sigma bonds in ... - Brainly.com All the bonds in the compound is single bond ( -bond) that is they are formed by head on collision of the orbitals. The structure of the compound is shown in the image. The Carbon-Hydrogen bond is formed by overlapping of s-orbital of hydrogen to p-orbital of carbon.

Label all bonds in so2
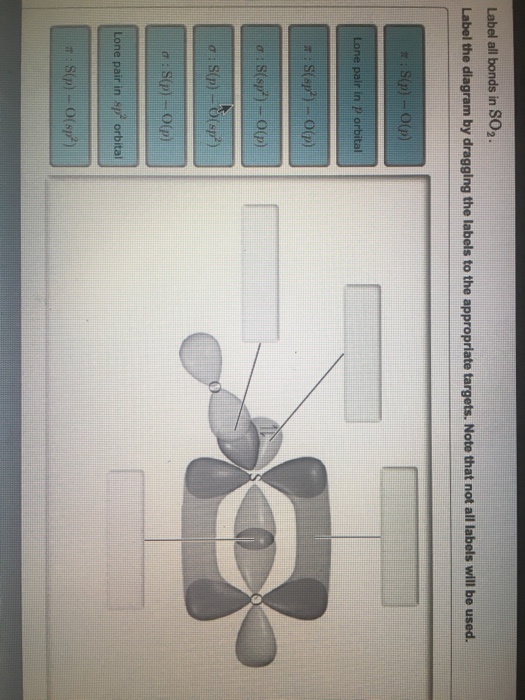
SOLVED:Write a hybridization and bonding scheme for each ... - Numerade Sketch the molecule, including overlapping orbitals, and label all bonds using the notation shown in Examples 6.1 and 6.2. a. CH2Br2 b. SO2 c. NF3 d. BF3. Answer (a) See solution (b) See solution (c) See solution (d) See solution. View Answer. Related Courses. Chemistry 101. Chemistry: Structure and Properties. Chapter 6. Chemical Bonding II. Solved In the sketch of the structure of SO2 label all | Chegg.com Transcribed image text: In the sketch of the structure of SO2 label all bonds. Drag the appropriate labels to their respective targets. Labels can be used once, more than once, or not Review Constants Periodic Table In the sketch of the structure of SO, label all bonds. Drag the appropriate labels to their respective targets. Solved Label all bonds in SO2. Label the diagram by dragging | Chegg.com Expert Answer Transcribed image text: Label all bonds in SO2. Label the diagram by dragging the labels to the appropriate targets. Note that not all labels will be used. a S (sp) -O (p) Lone pair in p orbital S (p) O (p) S (spa) O (p) Lone pair in sp orbital Submit My Answers Give U Previous question Next question
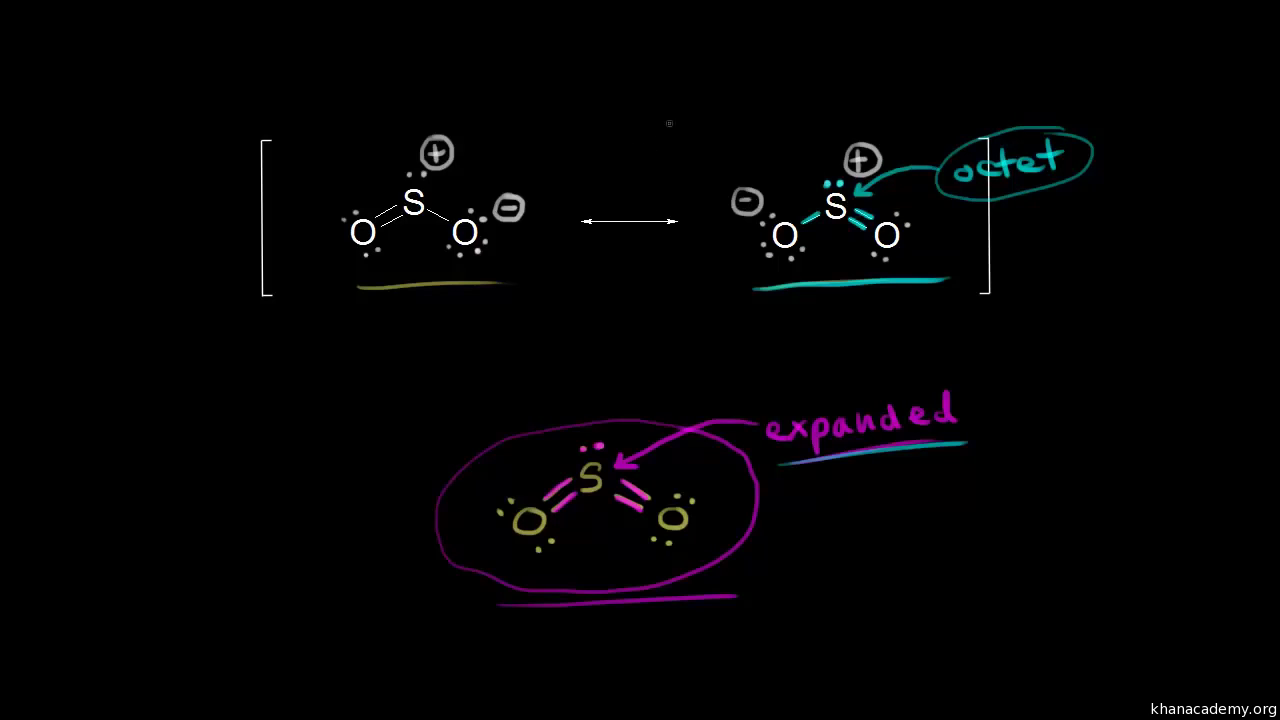
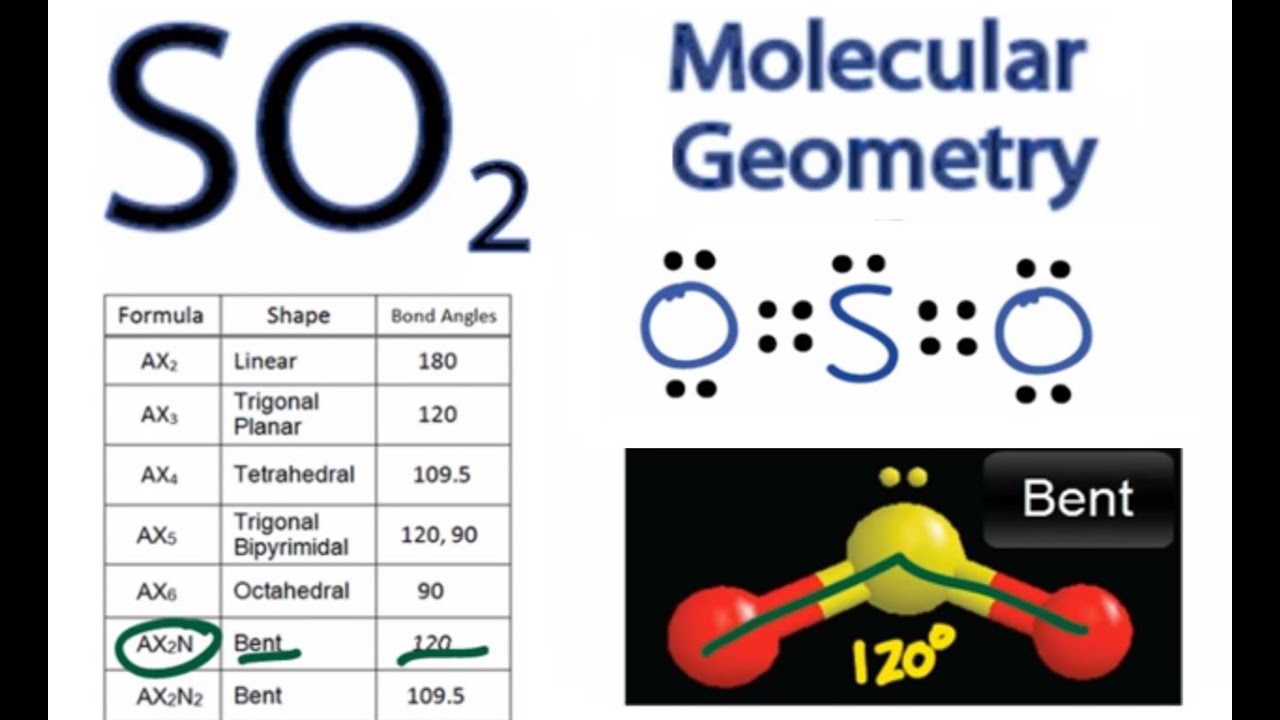
Label all bonds in so2. What is the hybridisation of sulphur dioxide? - Quora Sulphur dioxide is SO2 The central sulphur atom is bonded to two oxygen atoms. The structure is like this: O=S=O The sulphur atom forms one sigma and one pi bond with each oxygen atom and has one lone pair. Sulphur in its ground state has first two shells completely filled and six electrons in the outermost shell. What is the electronic geometry of SO2? - Quora Answer (1 of 4): Do you mean the shape of a sulfur dioxide molecule? Please re-write the question. If that is the question it is bent linear SO2 Lewis Structure, Hybridization, Molecular Geometry, and MO Diagram The molecular geometry of SO2 is bent, with a bond angle of 120°. We can easily find out the molecular geometry of any compound using the given chart. Here, A = central atom, X = surrounding atoms and E = the lone pairs. SO2 is an AX2E type molecule, with 2 surrounding atoms i.e oxygen, and 1 lone pair of sulfur. How is the molecular geometry for SO2 determined? - Quora Answer (1 of 10): "1. Explain the difference in polarity between CO2 and SO2 based ont heir molecular shape? SO2 is bent, so the two dipoles of the element-oxygen bonds do not cancel out like they do in CO2. Thus SO2 has a permanent dipole, and a relatively strong one. 2. Describe the similarit...
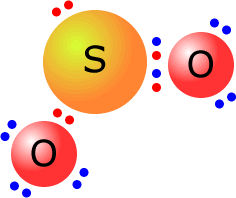
What is the lewis structure for SO_2? | Socratic 1. Decide which is the central atom in the structure. That will normally be the least electronegative atom ( S ). 2. Draw a skeleton structure in which the other atoms are single-bonded to the central atom: O-S-O. 3. Draw a trial structure by putting electron pairs around every atom until each gets an octet. inorganic chemistry - How does SO2 have 2 π bonds? - Chemistry Stack ... The hybridization of sulfur atom is sp2 hence a lone pair and two bond pairs (due to sigma bonding) reside in these hybrid orbitals. The unpaired electrons are 3p and 3d hybridized orbitals are used in pi bonding with oxygen's unhybridized 2p orbitals. Hence two pi bonds are formed which are p-p pi and d-p pi bonds. Label all bonds in CH2Br2. Label all bonds in SO2. Label all bonds in ... Label all bonds in SO2. Label all bonds in NF3. Label all bonds in BF3. Answer The molecule's name is CH2Br Br Br br dibromomethane. The molecule can be described as a derivative methane. The central atom of carbon is bonded with two hydrogen atoms, and two bromine. All bonds are sigma. Below is the electron configuration for the atoms. Answered: In the sketch of the structure of SO2… | bartleby In the sketch of the structure of SO2 label all bonds. Drag the appropriate labels to their respective targets. Labels can be used once, more than once, or not at all. : S (p) - O (p) Lone pair in p orbital Lone pair in sp? orbital o : S (p) - O (sp²) т: S (p) — О (p) т: S (sp?)
SO2 bond sigma and pi bond - CHEMISTRY COMMUNITY The Lewis Structure for SO 2 is a central Sulfur double bonded to each of the Oxygen atoms. A double bond consists of one sigma bond and one pi bond so in total you would have two sigma bonds and two pi bonds (one sigma and pi for one bonded Oxygen and another sigma and pi for the other). Top Sebastian Lee 1L Posts: 157 Answered: In the sketch of the structure of BF3… | bartleby Transcribed Image Text: In the sketch of the structure of BF3 label all bonds. Drag the appropriate labels to their respective targets. Labels can be used once, more than once, or not at all. Lone pair in sp orbital : B(p) - F(p) Empty p orbital Lone pair in p orbital B B(sp²) - F(p) в : В(8) — F(p) o : B(p) - F(p) Empty sp? orbital Solved Label all bonds in SO2. The hybridization of the S | Chegg.com Label all bonds in SO2. The hybridization of the S atom in SO2 is sp^2. Label all bonds in NF3. Hybridization of N atom in NF3 is sp^3 Show transcribed image text Expert Answer 80% (50 ratings) Transcribed image text: Label all bonds in SO Label the diagram by dragging the labels to the appropriate targets. organic chemistry: how many s-sp3 bonds? The correct answer is 13. I know that s-sp3 bonds are single bonds. But when I count them up, even the ones that aren't shown in the diagram I do not get 13 as theanswer. I get 12. Answer. The H-C bond s-sp3 is where the C has been sp3 hybridized. Conclusion. Above is the solution for "organic chemistry: how many s-sp3 bonds?".
SO2(Sulfur Dioxide) Lewis Structure, Hybridization, Molecular Geometry ... SO2 comprises a Sulfur atom surrounded by two oxygen atoms. In its most stable state, the Sulfur atom forms double bonds with the adjacent oxygen atoms. There is also a lone pair above the Sulfur atom. The hybridization of the SO2 is given by sp2. SO2 has a Bent molecular structure and shape with bond angles of 120°. About Priyanka
CHEM: Chapter 10 Flashcards | Quizlet The sp3 and sp3d2 hybridization schemes have no unhybridized p-orbitals left to form π-bonds. Valence Bond Theory 62. Write a hybridization and bonding scheme for each molecule. Sketch the molecule, including overlapping orbitals, and label all bonds using the notation shown in Examples 10.6 and 10.7. a. CH2Br2 b. SO2 c. NF3 d. BF3
(Solved) - Label all bonds in CH2Br2 Label the diagram by ... - Transtutors Label all bonds in ...
Sulfor dioxide: Lewis dot structure for SO2 (video) - Khan Academy The dot structure for sulfur dioxide has sulfur with a double bond to an oxygen on the left, and two lone pairs of electrons on that oxygen, and the sulfur with a double bond to an oxygen on the right, and two lone pairs of electrons on that oxygen. And then we have a lone pair of electrons on our sulfur.
SO2 Hybridization - YouTube A description of the hybridization of SO2 including sigma and pi bonds.Note that the SO2 hybridization is sp2 for the central sulfur atom. It's also sp2 fo...
Solved Label all bonds in SO2. Label the diagram by dragging | Chegg.com Expert Answer Transcribed image text: Label all bonds in SO2. Label the diagram by dragging the labels to the appropriate targets. Note that not all labels will be used. a S (sp) -O (p) Lone pair in p orbital S (p) O (p) S (spa) O (p) Lone pair in sp orbital Submit My Answers Give U Previous question Next question
Solved In the sketch of the structure of SO2 label all | Chegg.com Transcribed image text: In the sketch of the structure of SO2 label all bonds. Drag the appropriate labels to their respective targets. Labels can be used once, more than once, or not Review Constants Periodic Table In the sketch of the structure of SO, label all bonds. Drag the appropriate labels to their respective targets.
SOLVED:Write a hybridization and bonding scheme for each ... - Numerade Sketch the molecule, including overlapping orbitals, and label all bonds using the notation shown in Examples 6.1 and 6.2. a. CH2Br2 b. SO2 c. NF3 d. BF3. Answer (a) See solution (b) See solution (c) See solution (d) See solution. View Answer. Related Courses. Chemistry 101. Chemistry: Structure and Properties. Chapter 6. Chemical Bonding II.




















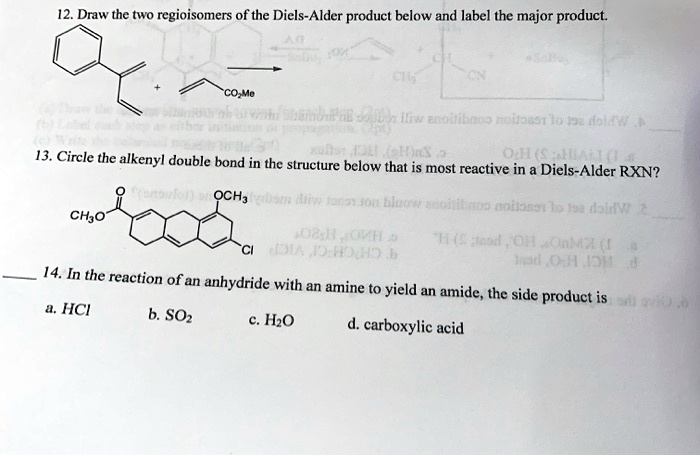
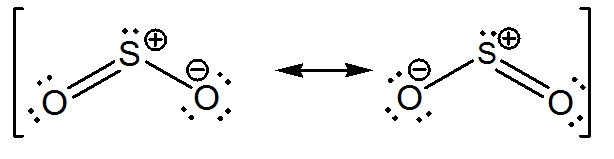

Post a Comment for "38 label all bonds in so2"