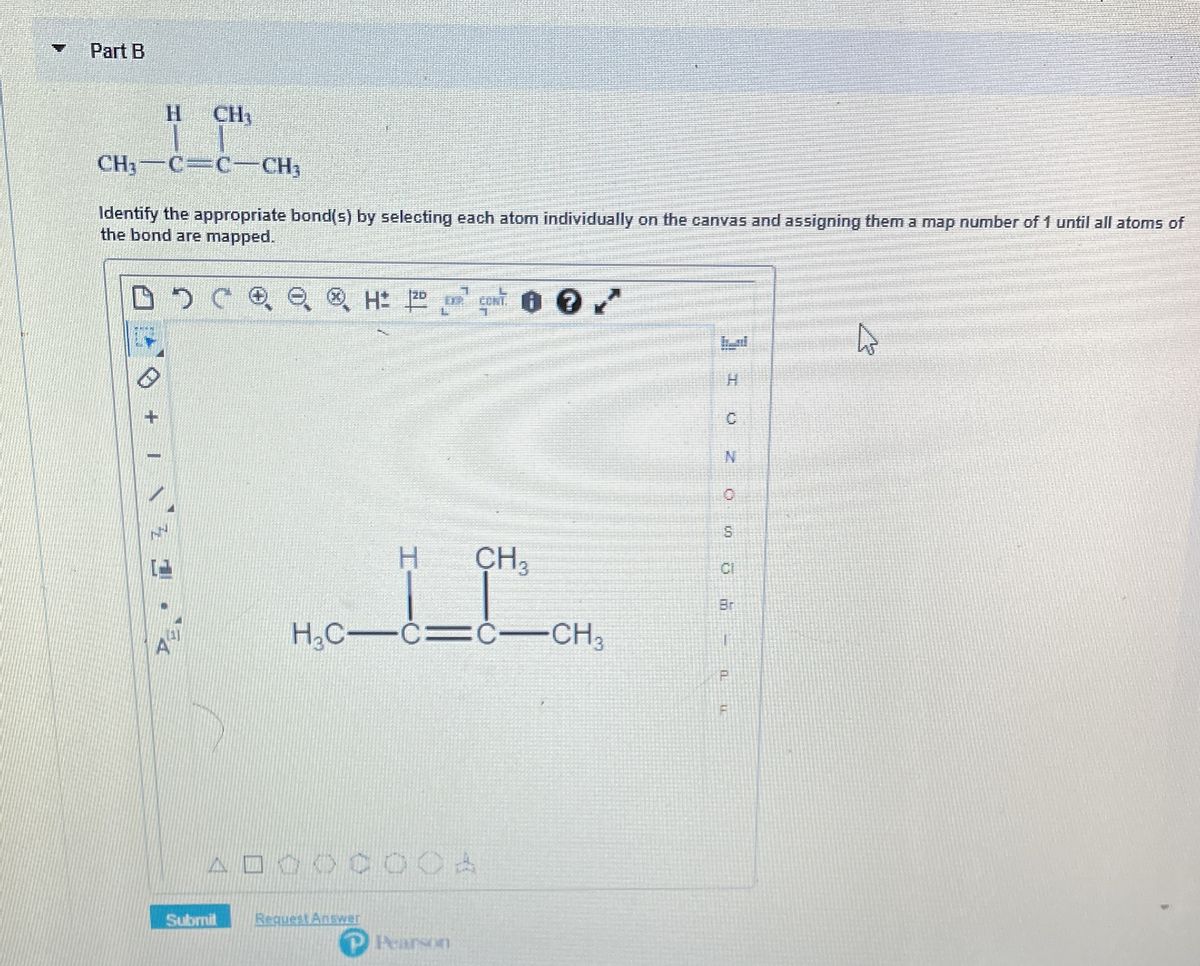
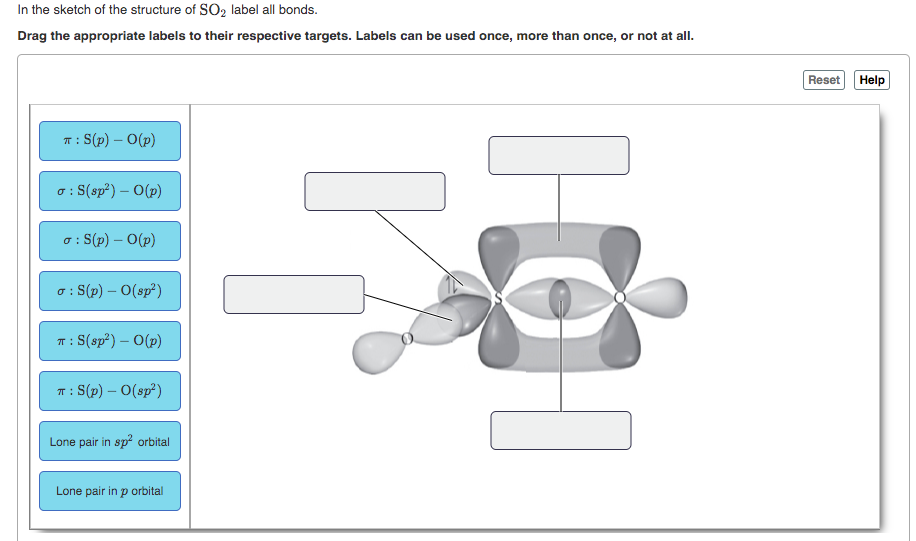
38 in the sketch of the structure of so2 label all bonds.
SOLVED:Write a hybridization and bonding scheme for each ... - Numerade Sketch the structure, including overlapping orbitals, and label all bonds using the notation shown in Examples 6.1 and 6.2. a. COCl2 (carbon is the central atom) ... we just need to account for the architect on flooring there. And now he's a label of these bonds. So the bonds from foreign are going to be S p three because they've got four ... CHEM: Chapter 10 Flashcards | Quizlet SO2 c. NF3 d. BF3 Valence Bond Theory 76. Using the molecular orbital energy ordering for second-row homonuclear diatomic molecules in which the p2p orbitals lie at higher energy than the s2p, draw MO energy diagrams and predict the bond order in a molecule or ion with each number of total valence electrons.
Chapter 7 Flashcards | Quizlet All of the following Lewis structures of nitrogen oxides are possible EXCEPT. N2O3. Which of the following is a correct Lewis structure for sulfur dioxide, SO2?:::O - :S -- O:: ... How many sigma and pi bonds are present in the following molecule?
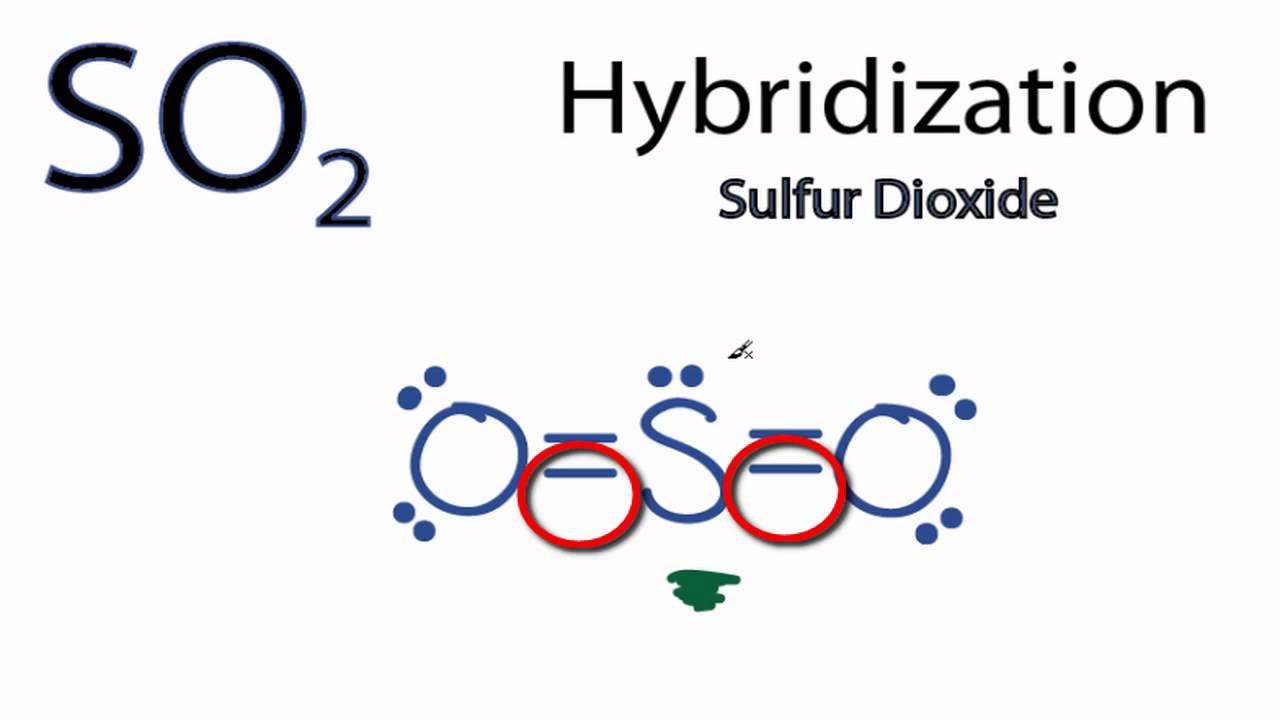
In the sketch of the structure of so2 label all bonds.
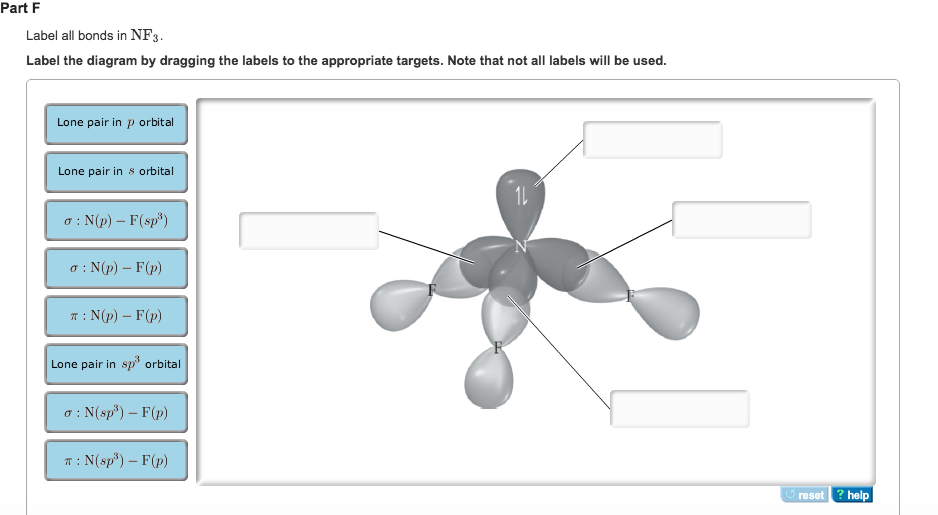
Solved In the sketch of the structure of CH Br2 label all | Chegg.com Labels can be used once, more than once, or not at all. 1 : S(sp) - 0(p) 0:S(sp) - 0(p) T: S(p) - 0(p) HO Lone pair in sp orbital 1 : S(p) - 0(sp) 0:S(p) - 0(p) 0:S) - O(sp) Lone pair in p orbital In the sketch of the structure of NF3 label all bonds. Drag the appropriate labels to their respective targets. Labels can be used once ... SOLVED:Write a hybridization and bonding scheme for each ... - Numerade Sketch the molecule, including overlapping orbitals, and label all bonds using the notation shown in Examples 6.1 and 6.2. a. CH2Br2 b. SO2 c. NF3 d. BF3 Joshua S. ... Chemistry is the science of matter, especially its chemical reactions, but also its composition, structure and properties. Chemistry deals with atoms and their interactions with ... Chapter 6, Chemical Bonding II Video Solutions, Chemistry: Structure ... Write a hybridization and bonding scheme for each molecule that contains more than one interior atom. Indicate the hybridization about each interior atom. Sketch the structure, including overlapping orbitals, and label all bonds using the notation shown in Examples 6.1 and 6.2. a. C2H2 (skeletal structure HCCH) b.
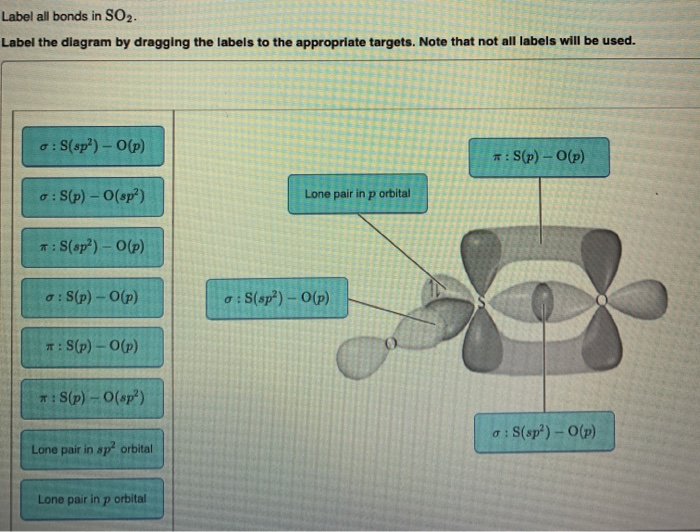
In the sketch of the structure of so2 label all bonds.. Solved Label all bonds in SO2. The hybridization of the S - Chegg Label all bonds in SO2. The hybridization of the S atom in SO2 is sp^2. Label all bonds in NF3. Hybridization of N atom in NF3 is sp^3 Show transcribed image text Expert Answer 80% (50 ratings) Transcribed image text: Label all bonds in SO Label the diagram by dragging the labels to the appropriate targets. Solved In the sketch of the structure of SO2 label all | Chegg.com Expert Answer 100% (42 ratings) Answer … View the full answer Transcribed image text: In the sketch of the structure of SO2 label all bonds. Drag the appropriate labels to their respective targets. Labels can be used once, more than once, or not at all. How is the molecular geometry for SO2 determined? - Quora SO2 is bent, so the two dipoles of the element-oxygen bonds do not cancel out like they do in CO2. Thus SO2 has a permanent dipole, and a relatively strong one. 2. Describe the similarities bewteen H3O+ and NH3. Compare/contrast their shapes and polarities within the context of your answer. These molecules are called isoelectronics. Why? Answered: In the sketch of the structure of BF3… | bartleby Transcribed Image Text: In the sketch of the structure of BF3 label all bonds. Drag the appropriate labels to their respective targets. Labels can be used once, more than once, or not at all. Lone pair in sp orbital : B (p) - F (p) Empty p orbital Lone pair in p orbital B B (sp²) - F (p) в : В (8) — F (p) o : B (p) - F (p) Empty sp ...
Solved In the sketch of the structure of CH2 Br2 label all - Chegg Expert Answer 100% (12 ratings) Transcribed image text: In the sketch of the structure of CH2 Br2 label all bonds. Drag the appropriate labels to their respective targets. Labels can be used once, more than once, or not at all. CO2 Lewis Structure, Molecular Geometry and Hybridization For Lewis structure of CO2, you will now have two Oxygen atoms forming double bonds with a Carbon atom. As all the valence electrons of all the atoms are used, there are no lone pairs of electrons or non-bonding pairs of electrons in the molecule. To further understand the molecular geometry of CO2, let us quickly go through its hybridization ... Sulfor dioxide: Lewis dot structure for SO2 (video) - Khan Academy The dot structure for sulfur dioxide has sulfur with a double bond to an oxygen on the left, and two lone pairs of electrons on that oxygen, and the sulfur with a double bond to an oxygen on the right, and two lone pairs of electrons on that oxygen. And then we have a lone pair of electrons on our sulfur. What is the lewis structure for SO_2? | Socratic Here are the steps I follow when drawing a Lewis structure. > 1. Decide which is the central atom in the structure. That will normally be the least electronegative atom ("S"). 2. Draw a skeleton structure in which the other atoms are single-bonded to the central atom: "O-S-O". 3. Draw a trial structure by putting electron pairs around every atom until each gets an octet. In this editor, I will ...
Solved In the sketch of the structure of SO2 label all | Chegg.com Question: In the sketch of the structure of SO2 label all bonds. Drag the appropriate labels to their respective targets. Labels can be used once, more than once, or not Review Constants Periodic Table In the sketch of the structure of SO, label all bonds. Drag the appropriate labels to their respective targets. (Get Answer) - For the SO2 molecule, a) Draw a Lewis structure that ... For the SO2 molecule, a) Draw a Lewis structure that satisfies the octet rule b) Determine the type of hybridization on the central atom c) Indicate both the electron geometry and the molecular geometry d) Draw an "orbital overlap diagram" showing all orbitals used in bonding e) Label all bonds as sigma or pi f) Label the bond angle about the central atom in your diagram g) Indicate whether ... Draw the Lewis structure for sulfur dioxide, SO2, which does not ... Arguably, one could construct a resonance structure where only the third lone pair on one oxygen forms a #pi# bond, but that is less stable of a resonance structure. The most stable resonance structure has two double bonds. And in fact, sulfur can do this because it has #3d# orbitals to utilize. Chapter 10: Chemical Bonding II: Molecular Shapes, Valence ... - Quizlet Begin by drawing the Lewis Structure (any resonance structure can be used to determine the number of electron groups) Based on the number of electron groups around the central atom, determine the geometry that minimizes the repulsions between electron groups ... Label all bonds using the σ or π notation followed by the type of overlapping ...
SOLVED:Write a hybridization and bonding scheme for each ... - Numerade We drew two bonds, um, or two bonds to each oxygen, which takes four of its electrons, and it has a total of six. So we have an extra lone electron pair here. So adding up the total things on the sulfur we see that's using to pure metals, Um, and two Sigma or Bills and the lone electron pairs. So this has to be S P three d.
What is the electronic geometry of SO2? - Quora Answer (1 of 4): Do you mean the shape of a sulfur dioxide molecule? Please re-write the question. If that is the question it is bent linear
SO2(Sulfur Dioxide) Lewis Structure ... - Geometry of Molecules SO2 Bond angles According to the VSEPR theory, the Oxygen atoms are repelled by each other and the lone pair, thus forming a bent molecular shape. As such, the bond angle of SO2 is 119°. SO2 Molecular Geometry and Shape To determine the molecular geometry of Sulfur Dioxide, we must observe its Lewis structure.
Lewis Structure of CH3- (With 6 Simple Steps to Draw!) These pairs of electrons present between the Carbon (C) and Hydrogen (H) atoms form a chemical bond, which bonds the carbon and hydrogen atoms with each other in a CH3 molecule. Step #4: Complete the octet (or duplet) on outside atoms. If the valence electrons are left, then put the valence electrons pair on the central atom.
SOLVED:Write a hybridization and bonding scheme for COCl2 ... - Numerade Answer Write a hybridization and bonding scheme for each molecule. Sketch the molecule, including overlapping orbitals, and label all bonds using the notation shown in Examples 10.6 and 10.7. a. C C l 4 b. N H 3 c. O F 2 d. C O 2 Discussion You must be signed in to discuss. Video Transcript So we're continuing on with molecular bonding.
Drawing structures - Bonding - OCR Gateway - BBC Bitesize For this, draw four circles, one labelled N and three labelled H. Each of the three H circles overlaps the N circle. It is often easiest to draw circles at 90° or 180° to each other Nitrogen is in...
Answered: In the sketch of the structure of SO2… | bartleby Question Transcribed Image Text: In the sketch of the structure of SO2 label all bonds. Drag the appropriate labels to their respective targets. Labels can be used once, more than once, or not at all.
SO2 Lewis Structure - How to Draw the Lewis Structure for SO2 ... - YouTube Find the total valence electrons for the SO2 molecule. 2. Put the least electronegative atom in the center. Note: Hydrogen (H) always goes outside. 3. Put two electrons between atoms to form a...
SO2 Lewis Structure, Hybridization, Molecular Geometry, and MO Diagram The molecular geometry of SO2 is bent, with a bond angle of 120°. We can easily find out the molecular geometry of any compound using the given chart. Here, A = central atom, X = surrounding atoms and E = the lone pairs. SO2 is an AX2E type molecule, with 2 surrounding atoms i.e oxygen, and 1 lone pair of sulfur.
Answered: The structure of acetylsalicylic acid… | bartleby A: To find the molar mass of compound , we have to take sum of all the masses of different elements pre... question_answer Q: CH3F is a polar molecule, even though the tetrahedral geometry often leads to nonpolar molecules.
Sulfur dioxide | SO2 - PubChem Sulfur dioxide | SO2 or O2S | CID 1119 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety/hazards/toxicity information, supplier lists, and more. National Institutes of Health. National Library of Medicine. National Center for Biotechnology Information ...
Chapter 6, Chemical Bonding II Video Solutions, Chemistry: Structure ... Write a hybridization and bonding scheme for each molecule that contains more than one interior atom. Indicate the hybridization about each interior atom. Sketch the structure, including overlapping orbitals, and label all bonds using the notation shown in Examples 6.1 and 6.2. a. C2H2 (skeletal structure HCCH) b.
SOLVED:Write a hybridization and bonding scheme for each ... - Numerade Sketch the molecule, including overlapping orbitals, and label all bonds using the notation shown in Examples 6.1 and 6.2. a. CH2Br2 b. SO2 c. NF3 d. BF3 Joshua S. ... Chemistry is the science of matter, especially its chemical reactions, but also its composition, structure and properties. Chemistry deals with atoms and their interactions with ...
Solved In the sketch of the structure of CH Br2 label all | Chegg.com Labels can be used once, more than once, or not at all. 1 : S(sp) - 0(p) 0:S(sp) - 0(p) T: S(p) - 0(p) HO Lone pair in sp orbital 1 : S(p) - 0(sp) 0:S(p) - 0(p) 0:S) - O(sp) Lone pair in p orbital In the sketch of the structure of NF3 label all bonds. Drag the appropriate labels to their respective targets. Labels can be used once ...




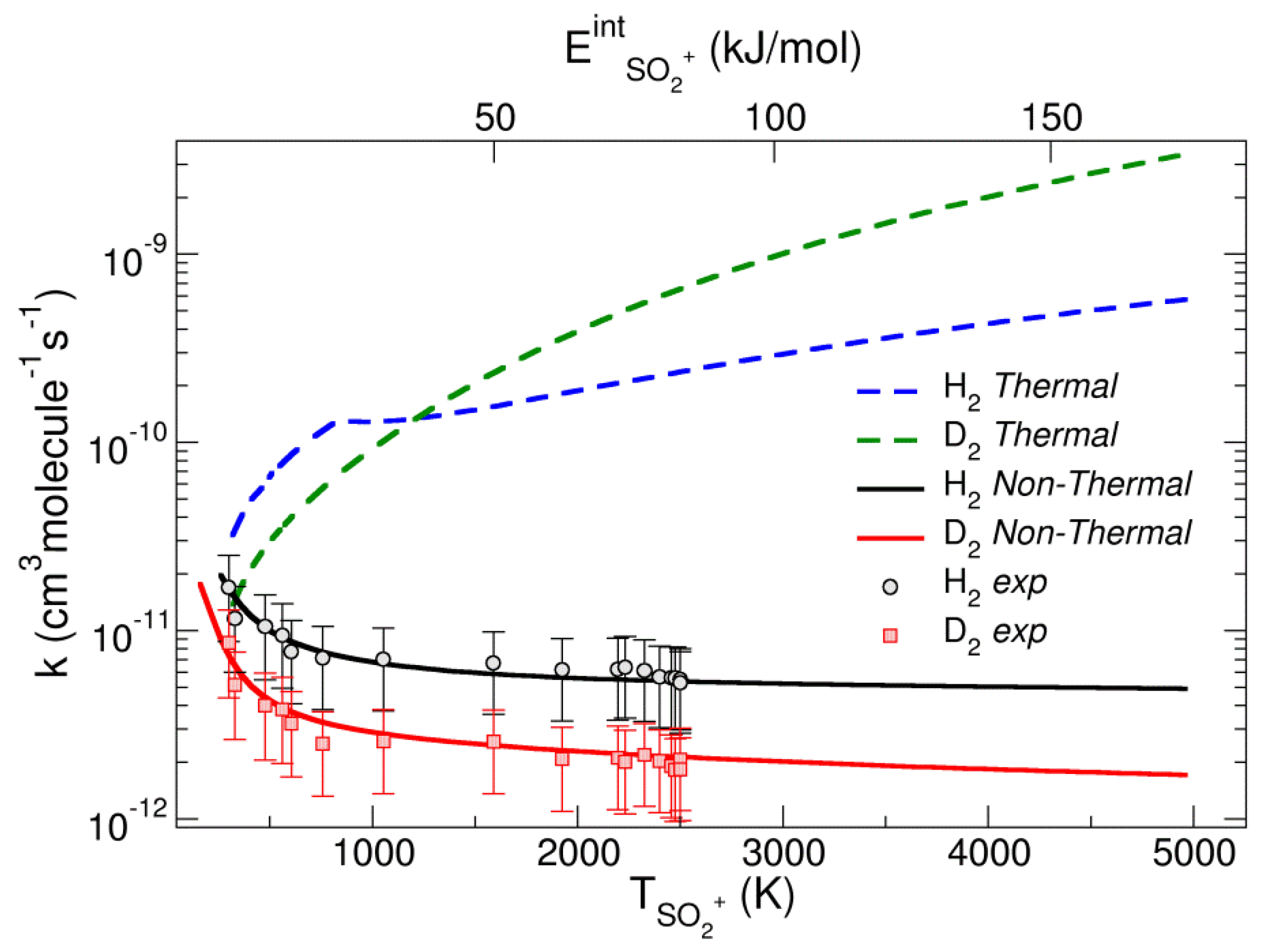
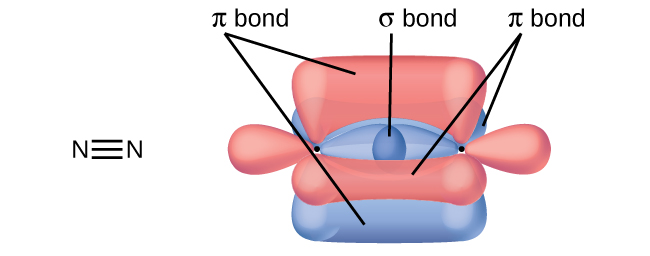
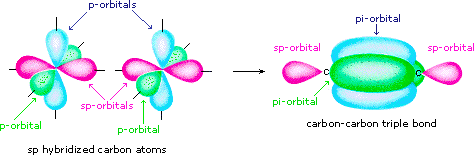










Post a Comment for "38 in the sketch of the structure of so2 label all bonds."